Holistic Detective Work: The Interconnectedness of Materials and Performance in Lateral Flow
Written by: Brendan O’Farrell of DCN Dx in partnership with Ahlstrom-Munksjö
“The term `holistic’ refers to my conviction that what we are concerned with here is the fundamental interconnectedness of all things. Let me give you an example. If you go to an acupuncturist with toothache he sticks a needle instead into your thigh. Do you know why he does that, Mrs Rawlinson?
No, neither do I, but we intend to find out.”
Douglas Adams
The proper performance of a lateral flow assay depends on the interconnected performance of a wide variety of materials, chemistries, biological reagents and processing steps. As a result, assay development and troubleshooting in manufacturing require a holistic approach. It is necessary to fundamentally understand the system with which we are working to isolate and fix problems and a willingness to suspend disbelief at times when attempting to do so. You’d be amazed at the things that can go wrong and the things that can work to fix them. There is rarely just one cause or just one solution to an issue. That’s why there is no substitute for experience when it comes to working with lateral flow systems. That’s why, having worked with this technology for close to 30 years now, it often feels to me like it wasn’t invented – more like it was discovered and allowed to evolve slowly on its own.
People have tried to generate algorithms for development of lateral flow products and recently applied advanced programming and AI to the task. Robots have been created to assist with the development process. Computer models of lateral flow systems have been generated. Design of Experiments (DOE) principles have been tried. While some benefits have accrued from these efforts, nothing has replaced the experience and intuition of a good lateral flow development scientist as yet.
When designing an assay, we start with first principles. Carefully evaluate the target performance requirements, understand the user needs and the application environment. Then begin the process of selection of materials and reagents. Each material used in an assay is specifically screened and selected in the context of every other material and reagent in the system. We know what each material needs to do, we know the pros and cons of the available selections and we have a basic menu from which we select and then we begin the process of developing the treatments we need to use to make them work. Nothing is selected in isolation.
Let’s take the example of sample collection pads and conjugate release pad materials, what they have to do and how they can impact your assay.
Understanding sampling in the context of small volumes and rapid assays is critical
For many applications traditional lateral flow formats are capable of providing sufficient sensitivity. However, there is a growing demand for sensitivity in many applications that approaches that of nucleic acid amplification and detection methods. Standard approaches to labeling and detection in lateral flow are unlikely to reach the required sensitivities for these applications. Quantification and ease of use for consumer applications are also growing in importance. Sample collection and preparation is one key to improved sensitivity and overall performance in many instances. It should be remembered that a large element of the appeal of lateral flow and other point of need assay systems is that they should provide where possible a complete “sample-to-answer” solution in a single step. It is therefore critical to consider the system as a whole, including the sample, the sampling method, the sample pre-treatment methodology and the concentration of analyte in the system. Analyte concentration can be a confounding factor either when it is too high or too low for detection, and sample treatment can and must be used to overcome related issues.
Sampling refers to the generation of a representative sample of an inhomogeneous object. This inhomogeneity presents a challenge to the success of the analytical method. As applied to highly sensitive rapid diagnostics, it is not the absolute sensitivity of the system that is the most critical factor, rather it is the ability to acquire as representative a sample as possible, and that, ultimately, it is the concentration of the analyte that one can detect in the primary sample that is critical. Sampling and pre-treatment methods, primarily concentration and the removal of potential cross reactive agents and reduction of background, are critical to determining the availability of many analytes for detection in an assay. Additionally, in certain circumstances, high concentrations of analyte can be a confounding factor in an immunoassay.
In any assay system to be deployed in a decentralized testing environment, the sample collection, treatment and delivery method must be simple, robust, foolproof, and ideally an integrated component of the test device. Minimal user dependent steps should be required.
Let’s look at fingerstick whole blood collection and plasma separation as an example.
The primary processing steps for the blood sample are
- metering of quantitative or semi quantitative amounts of blood from the fingerstick
- separation of plasma from the sample without significant hemolysis
- delivery of the sample, either neat, neat with a chase buffer, or pre-diluted, to the device
The creation of solutions that achieve several or all of these steps in an intuitive, user friendly way, is a constant challenge, and the needs of every assay system are different.
Plasma Separation and Delivery to the Test
Let’s assume a quantitative volume of fingerstick blood can be collected and delivered to the test. Plasma separation can be achieved in a variety of ways in rapid assays. The most common approach is to use filtration membranes as part of the strip architecture.
Many lateral flow type systems utilize an in-line blood separation membrane such as Ahlstrom-Munksjö Cytosep® membranes. These systems are relatively efficient, although they are limited in the volume of sample that they can handle based on useful surface area. Often the system will require either a pre-dilution of the sample or a chase/wash buffer to follow in order to wash the plasma clear of the membrane and to provide enough volume to wet the system completely.
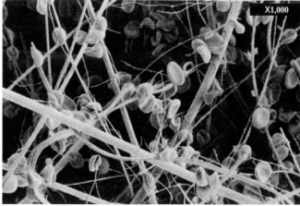
Filtration material for red blood cell separation
For many current and next-generation applications in lateral flow, quantification is important. So the ability to deliver a quantitative and highly reproducible amount of plasma to a test is one of the holy grails of this type of material. The volume of plasma that can be separated and delivered is of course confounded by hematocrit however the material should ideally be capable of accepting a defined volume of whole blood and delivering plasma to the test strip with consistent efficiency and reproducibility. There are other ways to address the hematocrit problem in the context of assays that are to be evaluated on reader systems.
Getting the plasma delivery right – minimizing hemolysis, maximizing reproducibility and separation efficiency, ensuring that analyte is not bound by the separation material and that the system flows in a timely and reproducible way – are the keys to success in this type of assay.
Conjugate Stabilization and Release
Switching to conjugate release pads, these materials – typically glass fibers and polyesters – can make or break your assay and your manufacturing process, and give you some gray hairs along the road.
The role of the conjugate pad in a typical assay system is to accept the conjugate, hold it stable over the shelf life of the product, and release it efficiently and reproducibly during that entire shelf life. In practical application, the variations in conjugate deposition, drying and release from the material demonstrably contribute the greatest sources of variation in assay performance, as measured by within and between lot coefficient of variation (CV). Assay sensitivity can also be adversely affected by poor conjugate mixing in, and release from, the conjugate pad. Depending on the system, it may be more important to achieve fast release or slow release of the conjugate, however release must always be consistent. Because of the nature of the materials used, it is often necessary to pre-treat conjugate pads to ensure the appropriate release and stability characteristics. Pad pretreatment is typically performed by immersion of the pad in an aqueous solution containing proteins, surfactants and polymers followed by drying. This process can be performed either in manual batch mode or in continuous inline mode, the latter giving the best opportunity for homogeneous processing of entire batches of materials.
The addition of conjugate to the treated pad is critical to the final performance of the test. Two methods are typically used:
- Immersion: where the treated conjugate pad is immersed in the conjugate suspension.
- Dispensing using quantitative non-contact dispensers such as the BioDot AirJet Quanti.
In relation to the conjugate system, the choices of label and conjugation methods are also critical. The most commonly used labels include colloidal gold, cellulose nanobeads and monodisperse latex, tagged with either a visual or a fluorescent dye. A variety of labels are now available, which can be covalently or passively coupled, and quantitatively read. Covalent coupling can be important to the ability to perform quantitative assays due to the inherently more stable bonds between the ligand and particle vs. typical passive adsorption methods.
The materials most commonly used are glass fibers, polyesters or rayons. The materials ideally should be hydrophilic and flow consistently. The materials used, however, are typically very hydrophobic in nature, and as a result must be treated to make them hydrophilic. This is typically done during the manufacturing of the assay rather than by the material manufacturer, although there are exceptions to that. This treatment involves the immersion of the pads in a solution of proteins, polymers and surfactants, followed by drying at high temperatures.
The goal is to use materials and treatments that enable efficient and reproducible release of the conjugate over the shelf life of the product. Typically some variation in release occurs due to binding of the particle conjugate to the fibers of the material. It is important during assay optimization to generate stabilization chemistries that minimize this effect and create the most efficient release of particles possible.
Finally, it is important that the material used should not destabilize the conjugate over entire shelf life (up to 2 years). Assay optimization therefore involves the testing of multiple materials for compatibility with the protein-particle conjugate being used.
The conjugate pad system is demonstrably responsible for the majority of variation in lateral flow assays when particulate labels are being used. This is due to inconsistency in the material, resulting in inconsistent uptake of the pretreatments and conjugates, destabilization of conjugates by binders, inconsistent release of the conjugate due to binding of particles to the hydrophobic fibers of the materials used. Great care must be taken in the optimization of the conjugates, the pad pre-treatment process and the conjugate deposition process to minimize these effects.
The interplay of these materials with each other and with the other materials in the assay will define how your assay and your manufacturing system will perform. Selecting materials from high quality suppliers who will work with you to provide the material characteristics and quality you need and who understand the interconnectedness of all things in the performance of the assay will be critical to success.
DCNovations is a line of high-quality precision rapid diagnostic test products and components distributed by DCN Dx, located in Carlsbad, California. DCN Dx is recognized globally as the go-to company for contract development and commercialization of rapid diagnostic tests. The company’s cross-functional team of scientists and engineers develop and integrate all aspects of assay systems including cassettes, sample handling devices, and reader systems. DCN Dx also provides services to researchers and labs that assist in the development of rapid diagnostic tests from concept to commercialization.